Modeling malaria using NetLogo
DISCLAIMER: I am not an epidemiologist. This article was created as part of a school project.
Exported HTML file of the NetLogo model can be found here.
Questions
The model aims to address the following questions:
- How effective would a malaria vaccine be in reducing the mortality rate of malaria?
- How effective is anti-malarial medication in reducing the mortality rate of malaria?
- How effective would combining the measures under questions 1 and 2 be in reducing the mortality rate of malaria?
Properties of the Disease
Malaria is a disease caused by Plasmodium parasites. The severity and frequency of episodes depends on the species of Plasmodium present in the body. Fortunately, the disease is curable if diagnosed and treated early and properly. (Centers for Disease Control and Prevention [CDC], n.d.).
While the parasites causing the disease are transmitted primarily through bites from infected female Anopheles mosquitoes, malaria is not contagious, i.e., it cannot be spread through regular physical contact with infected individuals. Transmission from blood transfusions is possible but avoidable with proper screening (Owusu-Ofori, Parry, & Bates, 2010).
Typically, the incubation period varies from 7 to 30 days before the first symptoms appear (CDC, n.d.). This period also varies between species of Plasmodium (Brasil et al., 2011).
After the incubation period, the infected person will experience symptooms such as fever, headache, and chills (World Health Organization [WHO], 2022). In some cases, commonly from infection with P. falciparum, the disease may induce complications and progress to severe malaria, requiring urgent medical attention due to its high mortality rate. If untreated, symptoms and attacks may recur at intervals ranging from days to months to years (CDC, n.d.).
As of writing, the only approved malaria vaccine is RTS,S, also known as Mosquirix, and it was approved for children aged 6 weeks to 17 months (GlaxoSmithKline, 2015).
Setup of the Simulation
The model was developed with NetLogo (Wilensky, 1999).
For the sake of simplicity, the model and simulations involve a selection of rules, limitations, and assumptions. These, however, come at the cost of real-world accuracy.
Sliders allow for the adjusting of rates and probabilities of certain parameters in the simulation.
Environment
- Each simulation or “run” begins with 50 human agents and 100 mosquito agents placed randomly.
- Each “tick” or frame in the simulation represents a day wherein interactions and progressions are computed.
- Each simulation runs for 120 ticks or days.
- Environment and terrain are not accounted for.
Mosquitoes
- Mosquito agents move randomly and do not seek human agents, only interacting when in close proximity.
- All mosquito agents can infect. Each mosquito agent has a 20% chance of biting at most one nearby human agent per day (de Almeida et al., 2010). Each bite has a 78% chance of infecting the human (Churcher et al., 2017).
- The number of mosquito agents remains constant.
Humans
- Human agents do not move in the simulation, based on the assumption that interactions with mosquitoes typically occur at home.
- Human agents do not reproduce, i.e., their population never increases.
- Human agents can only die while in the “severe” malaria state.
- Sick human agents can receive one unit of treatment per day from a limited pool proportional to the initial population as defined by input. Treatment has a 30% chance of failing and doing nothing (Lubell et at., 2014).
- Human agents in the “recovered” state cannot be infected for the rest of the simulation.
- Vaccinated human agents are marked as “recovered” at the start of the simulation, i.e., the vaccine provides complete immunity from infection and does not wane or expire within the duration of the simulation (Fuller, 2008).
Infections
- Infections are caused solely by mosquito bites.
- Upon infection, incubation time of the parasites is assigned a random value from a normal distribution with a mean of 13 days and a standard deviation of 3 days.
- After the parasites have incubated, the infected human agents become “sick” and have a 9% chance of progressing to severe malaria (Tekeste, Workineh, & Petros, 2012) within the day. Otherwise, they return to the “infected” state on the next day, resetting the incubation time to a random value between 2 to 4 days (CDC, n.d.).
- Humans with severe malaria have a 20% chance of dying each day and can only recover through medication. (Blumberg, Lee, Lipman, & Beards, 1996)
Given the aforementioned assumptions, the following scenarios were simulated: - No vaccinations and medication available - 25%/50%/75% of the population is vaccinated; no medication available - No vaccinations available; 25%/50%/75% medication available - Combinations of vaccination and medication (25%/50%/75%)
Each scenario was simulated 10 times via NetLogo’s BehaviorSpace tool.
Discussions
The simulations yielded the following results:
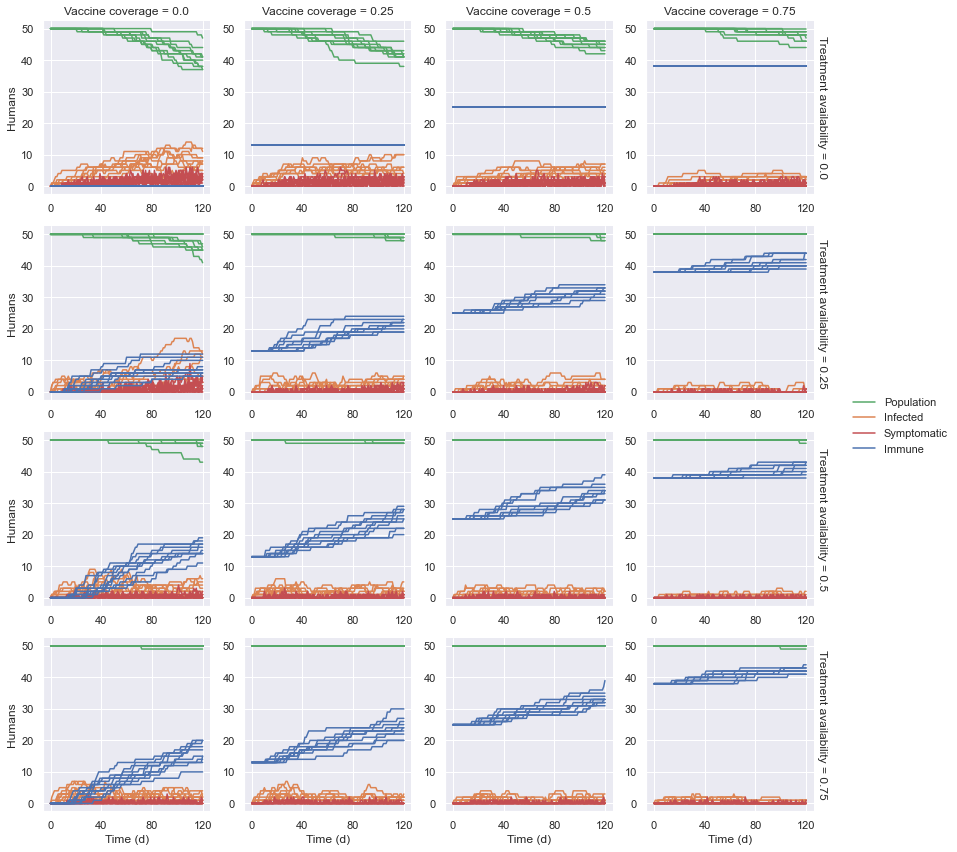
Assuming no medication available, the number of infections and, in turn, deaths decreased as the vaccine coverage increased. At 75% coverage, the number of deaths were roughly half of those without vaccines. Though the vaccines reduced the number of susceptible human agents, those infected were doomed to die still.
Assuming no vaccine coverage, the number and duration of infections, and in turn the number of deaths decreased as medication availability increased, to greater effect than with vaccines alone. At 75%, deaths were almost nonexistent. In general, the deaths occurred only after the supply was exhausted, i.e., the number of immune agents plateaued.
Combining these measures, however, provided significantly more positive results as deaths were greatly reduced starting with only 25% vaccine coverage and 25% medication availability, outperforming 50% of each without the other. Effectiveness was still increased as these measures were increased accordingly, though improvements were arguably not cost-effective. Even at maximum coverage (75%/75%), one death happened, hinting that even small lapses can result in losses.
Key Insights
Based on the results from the simulations, it can be said that:
- A malaria vaccine would be effective in reducing the mortality rate of malaria.
- Anti-malarial medication would be effective in reducing the mortality rate of malaria.
- Combining the two aforementioned measures would be effective in reducing the mortality rate of malaria and would produce better results than focusing on one measure alone.
Of note, however, is that each scenario was simulated only once and more simulations are required to reinforce the results.
References
Balentine, J. R., & Davis, C. P. (2018, October 3). How long will it take to recover from malaria? MedicineNet. Retrieved May 12, 2022 from https://www.medicinenet.com/how_long_will_it_take_to_recover_from_malaria/ask.htm
Blumberg, L., Lee, R. P., Lipman, J., & Beards, S. (1996, April 1). Predictors of mortality in severe malaria: A two-year experience in a non-endemic area. Anaesthesia and Intensive Care, 24(2), 217–223. Retrieved May 13, 2022 from https://doi.org/10.1177/0310057X9602400213
Brasil, P., de Pina Costa, A., Pedro, R. S., da Silveira Bressan, C., da Silva, S., Tauil, P. L., & Daniel-Ribeiro, C. T. (2011). Unexpectedly long incubation period of Plasmodium vivax malaria, in the absence of chemoprophylaxis, in patients diagnosed outside the transmission area in Brazil. Malaria journal, 10(122). Retrieved May 12, 2022 from https://doi.org/10.1186/1475-2875-10-122
Centers for Disease Control and Prevention. (n.d.). About Malaria - Disease. Retrieved May 6, 2022 from https://www.cdc.gov/malaria/about/disease.html
Centers for Disease Control and Prevention. (n.d.). About Malaria - Frequently Asked Questions (FAQs). Retrieved May 6, 2022 from https://www.cdc.gov/malaria/about/faqs.html
Churcher, T. S., Sinden, R. E., Edwards, N. J., Poulton, I. D., Rampling, T. W., Brock, P. M., Griffin, J. T., Upton, L. M., Zakutansky, S. E., Sala, K. A., Angrisano, F., Hill, A. V., & Blagborough, A. M. (2017). Probability of transmission of malaria from mosquito to human is regulated by mosquito parasite density in naïve and vaccinated hosts. PLoS pathogens, 13(1), e1006108. Retrieved May 13, 2022 from https://doi.org/10.1371/journal.ppat.1006108
de Almeida, S. J., Ferreira, R. P. M., Eiras, A. E., Obermayr, R. P., & Geier, M. (2010). Multi-agent modeling and simulation of an Aedes aegypti mosquito population. Environmental Modelling & Software, 25(12), 1490-1507. Retrieved May 13, 2022 from https://doi.org/10.1016/j.envsoft.2010.04.021
Delgado, M. L. V. (2020, April 24). An agent based model to assess malaria transmission drivers in the Ecuadorian Amazon [Master’s project]. Duke University. Retrieved May 6, 2022 from https://hdl.handle.net/10161/20508
Evans, D. R., Higgins, C. R., Laing, S. K., Awor, P., & Ozawa, S. (2019). Poor-quality antimalarials further health inequities in Uganda. Health policy and planning, 34(Supplement_3), iii36–iii47. https://doi.org/10.1093/heapol/czz012
Fuller, A. D. (2008, June). An agent-based model to study the spread and control of epidemics [Master’s project]. Virginia Commonwealth University. Retrieved May 12, 2022 from https://doi.org/10.25772/EX9Q-1T29
GlaxoSmithKline Biologicals S.A. (2015, July 23). Mosquirix: Opinion on medicine for use outside EU. European Medicines Agency. Retrieved May 11, 2022 from https://www.ema.europa.eu/en/opinion-medicine-use-outside-EU/human/mosquirix
Gharakhanlou, N. M., Hooshangi, N., & Helbich, M. (2020). A spatial agent-based model to assess the spread of malaria in relation to anti-malaria interventions in Southeast Iran. ISPRS International Journal of Geo-Information 2020, 9(9), 549. Retrieved May 8, 2022 from https://doi.org/10.3390/ijgi9090549
Howes, R. E., Franchard, T., Rakotomanga, T. A., Ramiranirina, B., Zikursh, M., Cramer, E. Y., Tisch, D. J., Kang, S. Y., Ramboarina, S., Ratsimbasoa, A., & Zimmerman, P. A. (2018). Risk factors for malaria infection in Central Madagascar: Insights from a cross-sectional population survey. The American Journal of Tropical Medicine and Hygiene, 99(4), 995-1002. Retrieved May 6, 2022 from https://dx.doi.org/10.4269%2Fajtmh.18-0417
Lubell, Y., Dondorp, A., Guérin, P. J., Drake, T., Meek, S., Ashley, E., Day, N. P., White, N. J., & White, L. J. (2014). Artemisinin resistance - modelling the potential human and economic costs. Malaria journal, 13(452). https://doi.org/10.1186/1475-2875-13-452
Owusu-Ofori, A. K., Parry, C., & Bates, I. (2010). Transfusion-transmitted malaria in countries where malaria is endemic: A review of the literature from Sub-Saharan Africa. Clinical Infectious Diseases, 26(10), 1192–1198. Retrieved May 12, 2022 from https://doi.org/10.1086%2F656806
Tekeste, Z., Workineh, M., & Petros, B. (2012). Determining the severity of Plasmodium falciparum malaria in Ethiopia. Journal of Infection and Public Health, 6(1), 10-15. Retrieved May 12, 2022 from https://doi.org/10.1016/j.jiph.2012.09.016
Wilensky U. (1999). NetLogo (Version 6.2.2) [Computer software]. Northwestern University. Evanston, IL: Center for Connected Learning and Computer-Based Modeling, Northwestern University. Retrieved May 13, 2022 from https://ccl.northwestern.edu/netlogo/
World Health Organization. (2022, April 6). Malaria. Retrieved May 13, 2022 from https://www.who.int/en/news-room/fact-sheets/detail/malaria